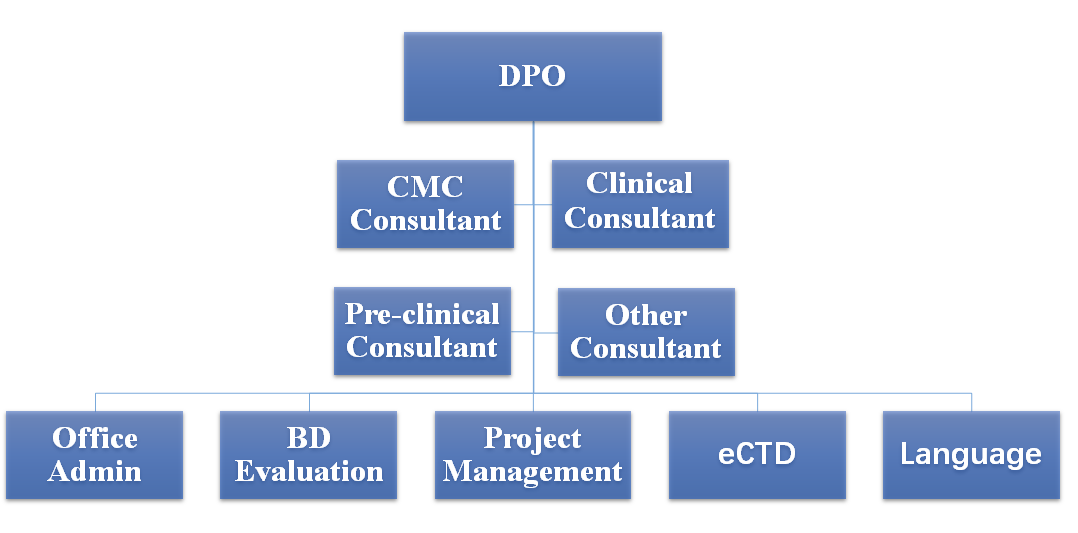
Humphries's team is structured into three levels: Chief Consultants, Professional Consultants, and Support Teams. Both Chief Consultants and Professional Consultants are experienced experts in the pharmaceutical industry, covering areas such as Chemistry, Manufacturing, and Controls (CMC), pharmacology/toxicology, clinical pharmacology, clinical trial design, medicine, statistics, Good Laboratory Practice (GLP), Good Clinical Practice (GCP), current Good Manufacturing Practice (cGMP) inspections, and audits, among others. The core consultants at Humphries have previously served as senior review officers at the U.S. Food and Drug Administration (FDA) and have in-depth knowledge of FDA approval processes and regulations, including Investigational New Drug (IND) applications, New Drug Applications (NDA), Biologics License Applications (BLA), and Abbreviated New Drug Applications (ANDA). Many consultants have also held technical executive positions in large and medium-sized pharmaceutical companies and Contract Research Organizations (CROs) in the United States, leading the research and development, clinical trials, and FDA registration of numerous candidate drugs. Humphries's support team encompasses sub-teams in project management, business assessment, language editing, electronic submissions, and more. The stable and professional core team, along with the comprehensive support system, ensures that Humphries can provide customers with continuous, stable, and high-quality consulting services.