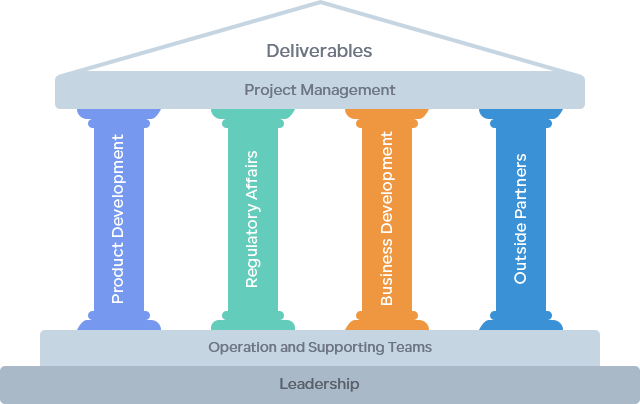
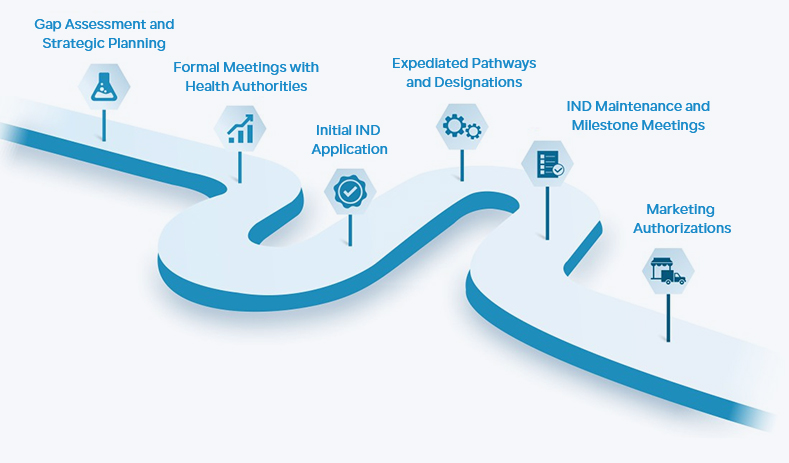
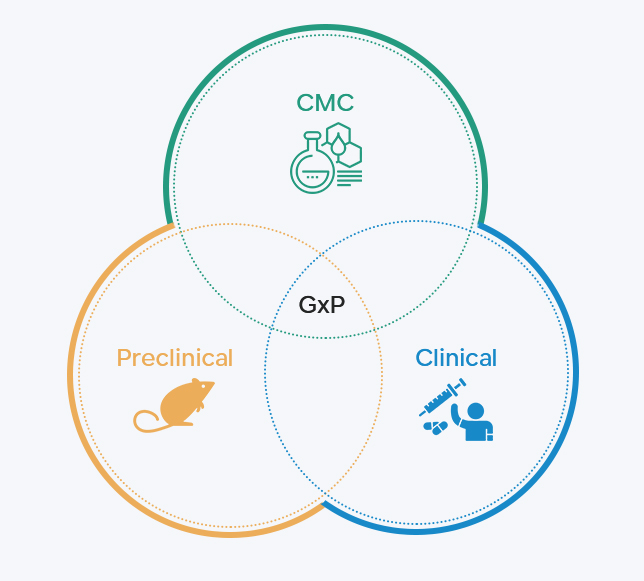
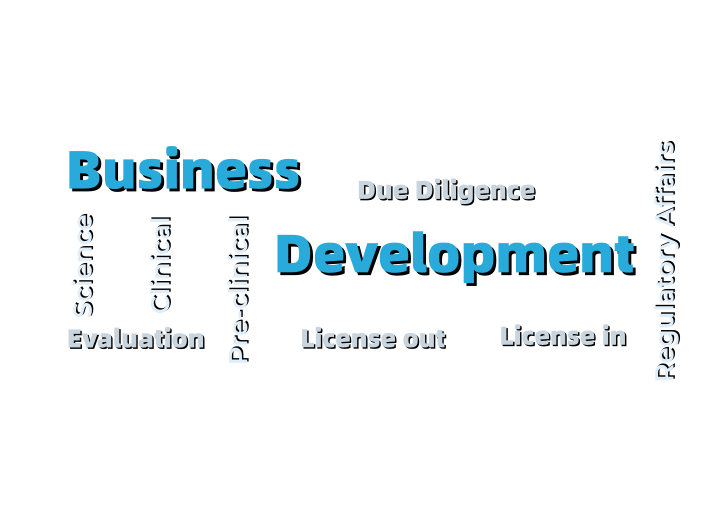
Humphries Pharmaceutical Consulting, abbreviated as HPC, is a professional consulting company that provides high-end regulatory services to the pharmaceutical industry. Founded in 2003 in Maryland, USA, and expanding to Nanjing, China, in 2012, HPC has gradually established a service system centered around pharmaceutical regulations, with drug registration, product development and compliance, business development, and project management as its pillars. HPC focuses on the process, commits to results, and works diligently. It accompanies the growth of Chinese pharmaceutical enterprises and contributes its efforts to the internationalization of the Chinese pharmaceutical industry.